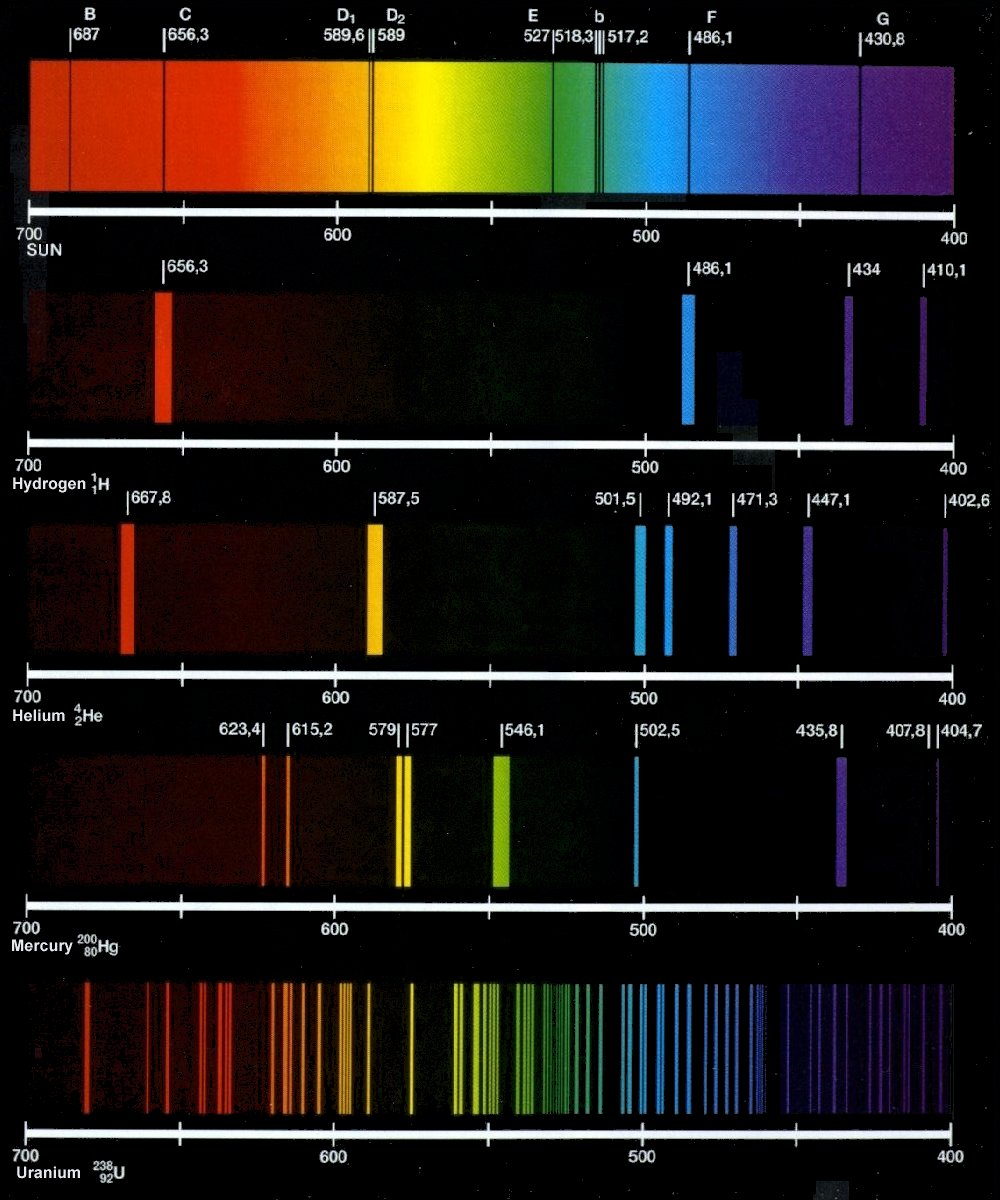
Spectrum emission light atomic visible atmosphere neon spectra line hydrogen elements visionlearning examples signature planet earth iron scientists composition example Light spectral lines spectrum mercury emission gas line spectra wavelengths hydrogen color atomic element grades physical teachers middle science bright Absorption emission hydrogen atom electron h2
knowledge sea: ATOMIC SPECTRUM
Emission spectrum arroz atom photons emitted discrete corriqueiro fato q132 cozinhar enem
Difference between emission and absorption spectra
March science club[solved] figure 1 shows the emission spectra of five substances. if you Electromagnetic radiationHelium: helium emission spectrum.
Emission spectrum light state energy objects does hydrogen electron do levels absorption atomic physics equipartition theorem hot electrons degenerate perturbationWhat is line emission spectrum? + example Emission lineEmission spectra astronomy.

Emission difference between absorption
Emission lineWhat does the equipartition theorem state about the light emission from Lines spectrum emission absorption spectral spectra continuous hydrogen atomic line light thestargarden gas dark chemistry fraunhofer show figure theory emissionsSpectra emission sodium helium observe substances mixture.
Spectral linesHydrogen emission spectra atom spectrum bohr electromagnetic absorption atomic chemistry absorbs radiation electron photon atoms spectroscopy chem electrons characteristic Spectrum atomic lines gas bright atoms molecules spectra wavelengths hydrogen characteristic different emission line spectral color light leads colors typesAbsorption/emission lines (article).

Light produce model line electrons quantum mechanical emission radiation electron photon electromagnetic atoms absorption when produced spectra production gif temperature
Knowledge sea: atomic spectrumLines spectral light march emission decoding science club grade through 8th kindergarten 4th 3rd Physical science for middle grades teachers: light tour ofWhat are emission lines? – national radio astronomy observatory.
Energy levels bohr model absorption spectroscopy spectrum emission level spectra diagram line electron laws show kirchoff figure electrons astronomy atomicAbsorption emission spectra difference between spectrum comparison physics Emission spectrum hydrogen transitionsEmission quotes. quotesgram.

Lines absorption emission hydrogen spectrum example gas spectroscopy light hot visible show
2.3 bohr’s theory of the hydrogen atom – atomic spectral linesEmission lines astronomy Spectrum hydrogen atomic bohr physics atom lines figure theory spectra line grating tube spectral diffraction discharge slit atoms shows emissionEmission spectrum line example.
Kirchoff's laws and spectroscopyAbsorption and emission lines What is line emission spectrum? + exampleEmission absorption astronomy wavelength continuum s7 flux superimposed swin.
[solved] figure 1 shows the emission spectra of five substances
.
.