How to draw the lewis dot structure for al2(so4)3 Dysocjacja jonowa soli. k3 po4 na2 so4 al2 (so4)3 k2 co3 ca cl2 k2 s ca 16% al2(so4)3 17% iron free aluminium sulphate for water treatment
How to Draw the Lewis Dot Structure for Al2(SO4)3 - YouTube
Mass so4 al2 aluminium obtained maximum 100g much
So4 al2 lewis draw
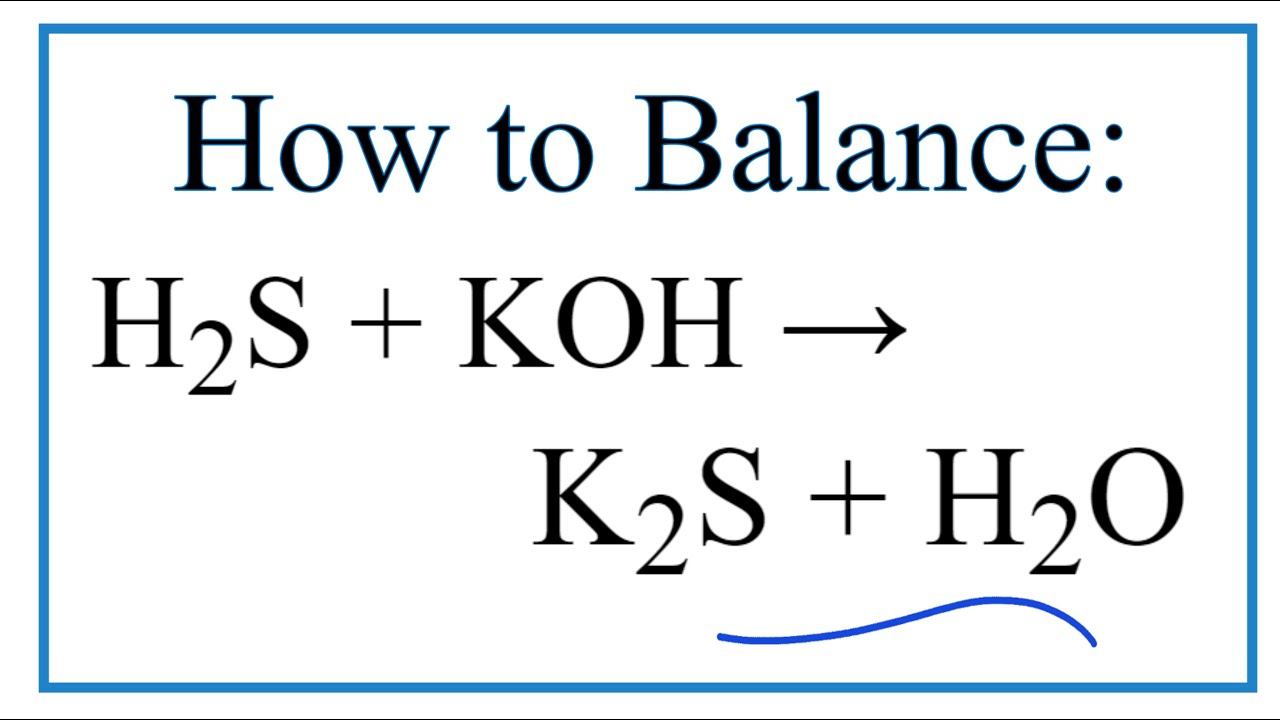
Given the unbalanced equation: ___al2(so4)3+___ca(oh)2—>___al(oh)3How to balance h2s + koh = k2s + h2o So4 al2 sulphate aluminum salt inorganic chemical materials diytrade chemicalsAluminium sulfate, al2 (so4) 3, for water treatment.
Aluminum sulphate / al2(so4)3So4 caso4 al2 unbalanced given So4 h2 al2 sulfuric h2o hno3 hcl o2 sodium h2s no3 sucrose fecl3 ph3 decomposition phosphoric so2 peroxide nano2 naohSo4 al2 sulfate aluminium water treatment china sulphate.

Chemical balancing of al2[so4]+naoh=al[oh]3+na2so4
Jonowa soli so4 brainly chemia al2 po4How much maximum mass of aluminium can be obtained from 100g of al2(so4 .
.



![chemical balancing of Al2[SO4]+NaOH=Al[OH]3+Na2SO4 - Brainly.in](https://i2.wp.com/hi-static.z-dn.net/files/de9/d9ee1dad3e9079379842c5027e741a4f.jpg)


